Pretty Pills, Please - The FDA's Focus on Generic Drug Appearance Creates Other Concerns
The FDA recently issued final guidance regarding the size, shape, and other physical characteristics of generic-manufactured tablet and capsule dosage forms. The guidance noted that differences in physical characteristics of a dosage form could affect patient compliance and acceptability of medication regimens, or could lead to medication errors. The main reason for the FDA’s guidance appears to be that many patients can experience difficulty swallowing tablets and capsules. But these issues can create impacts on the cost and availability of generic drugs, and may require an additional class of patents to be evaluated in clearing third-party patent rights when filing an ANDA and certain 505(b)(2) applications. For example, a larger tablet is harder to swallow than a smaller tablet, and oval tablets may be easier to swallow than round tablets. Other physical attributes that may affect a patient’s ability to swallow, and thus patient compliance with a recommended dosing regimen, include coatings, weight, surface area, disintegration time, and propensity for swelling.
Accordingly, the FDA’s guidance recommends that generic oral tablets and capsules intended to be swallowed intact should be of a similar size to the corresponding referenced listed drug (RLD). The FDA further recommends that tablets and capsules should have a similar shape or have a shape that has been found easier to swallow compared with the shape of the RLD. Moreover, other physical attributes of tablets and capsules should be considered in the context of their effect on ease of swallowing.
Many generic pharmaceutical enterprises have been opposed to the guidance because it proposes to eliminate an option that presently exists to design around a brand company’s patents directed to physical attributes. These attributes, such as tablet or capsule sizes, shapes, weights, surface areas, and disintegration times, are alleged to be patentable. Brand companies typically file patent applications directed to compositions, formulations and methods, but will sometimes file applications claiming physical attributes of the dosage form. Generic companies have previously been able to approach this type of patent through design around to avoid infringement, such as by altering the size and/or shape of the generic dosage form while still adhering to the requirement for bioequivalence to the brand name drug. The proposed guidance would limit this option in many cases, requiring generic applicants to engage in a typically more costly process to invalidate such patents directed to physical attributes.
U.S. Patent No. 8,383,152 (the “’152 patent”) is an example of a patent that claims a very specific shape of the dosage form. The ’152 patent is not listed in the Orange Book at present, but it claims dosage forms having different breaking strengths along different axes of the dosage form. Many of the dependent claims recite a particular shape of the dosage form. For example, claim 2 recites a shape having a longitudinal axis and two opposite longitudinal edges, a transversal axis perpendicular to the longitudinal axis and two opposite transversal edges, a front side, an opposite back side and a circumferential rim between the front and back side.
The front side and/or the back side include a basis area, and include at least one bulge that extends above the basis area. The “at least one bulge” is present at and/or adjacent to at least a section of one or both longitudinal edges and/or at and/or adjacent to at least a section of one or both transversal edges and/or between both longitudinal edges and both transversal edges. Figures 1A and 1B illustrate one embodiment of the shape.
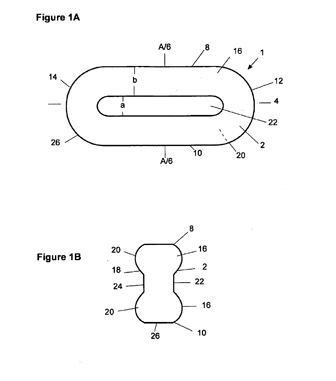
Generic Drug Shape Figures 1 and 2
To the extent that this patent ever lists for a product in the Orange Book, the ability to design around may be impacted by the FDA’s guidance. We would note, however, that the FDA guidance permits design around options that are expected to be easier to swallow.
Another physical attribute claimed as patentable by some brand companies is weight of the active pharmaceutical ingredient (API) relative to the dosage form. For example, U.S. Patent No. 6,294,197 (“the ’197 patent”) is listed in the Orange Book for several Novartis valsartan products. The ’197 patent claims a dosage form that includes an API present in an amount of more than 35 percent by weight based on the total weight of the dosage form. One way generics might attempt to get around this claim is to include a larger amount of pharmaceutical excipients so that the amount of API is less than 35 percent by weight of the dosage form, while still ensuring bioequivalence to the FDA’s specifications. This, however, tends to lead to a larger dosage form, which is now discouraged by the FDA.
Yet another attribute brand companies pursue is coatings. U.S. Publication No. 2013/0034605 (“the ’605 publication”) attempted to claim extended-release paliperidone compositions with various coatings. For example, claim 1, as published, recited a paliperidone core with a coating that includes a seal coating layer, a controlled release coating layer, a pH dependent polymer coating layer, and an optional overcoating layer. The ’605 publication was abandoned after the first Office Action. Another example is U.S. Publication No. 2010/0330180 (“the ’180 publication”), which tried to claim a process and tablet that included a specific coating layer coated with an anti-static agent. The anti-static agent was recited as being selected from the group consisting of kaolin, magnesium trisilicate, starch, microcrystalline cellulose, bentonite, silicon dioxide, cellulose, stearic acid, sodium stearyl fumarate, and glycerol behenate. The ’180 publication was abandoned after the first Office Action and an Examiner interview.
Ultimately, the FDA guidance may aid a subclass of patients swallow their medications (while also providing additional motivation for making certain design choices regarding particular patent-seeking approaches potentially obvious). But this limits a non-infringement paragraph IV certification option on patents directed to physical attributes, and resolves the costly Hatch-Waxman/ANDA disputes that more often arise after invalidity certifications, appears to overlook the likely increased generic drug costs when physical attribute patents are listed in the Orange Book or exist as unlisted roadblocks to marketing. Indeed, if health-care consumers, insurers, and budget-conscious governments are concerned about generic drug pricing (which affects ultimate access to the medication) and shortages, this proposed guidance is not likely to help resolve such ongoing problems in the drug supply chain.
Please feel free to contact the authors to learn more about the patent issues involved in launching an ANDA or 505(b)(2) product when designing around or avoiding brand company patents. The FDA Guidance may be found here.
Link to article


 Post a comment
Post a comment Print article
Print article

